Developing a broad pipeline of precision dermatology products
Program Areas & Pipeline
Azitra is using its Microbiome Dermatology Platform to develop products to improve skin appearance and treat skin diseases through three program areas:
Program Area #1: Consumer Products
Azitra’s technology can be applied to address many consumer needs related to skin appearance. Such products will be applied as topical ointments and creams to address conditions including: dry, red, flaky or inflammed skin.
Azitra and Bayer have formed a joint development program targeted at harnessing the human skin microbiome as a source for new natural skin care products for sensitive and eczema-prone skin. To learn more about the collaboration, read the press release here.
Program Area #2: Live BioTherapeutic Products (LBP’s) for Treating Skin Disease
Azitra is using the natural commensal bacteria Staphylococcus epidermidis (SE) from the skin microbiome to develop bioengineered microbiome products for the treatment of skin disease.
Program Area #3: Advanced Technology Indications based on the Microbiome
Azitra has received grant funding from U.S. Department of Defense (DoD) and other agencies to develop advanced microbiome products for military, public health and consumer applications. The overall program includes candidates that target Staph aureus (including MRSA) to reduce potential for mosquito biting and consequently reduce the potential of mosquitos to promote disease vectoring. The Company is also studying the use of commensal bacteria for use as biosensors which may have application in targeting adverse organisms that may attack the skin and potentially for use to counter bio-hazards.
ATR-12
Discovery
Disc.
Preclinical
P.C.
Phase 1
Ph.1
Phase 2
Ph.2
Phase 3
Ph.3
ATR-12
Netherton Syndrome (Pediatric Rare Disease Designation)

Netherton syndrome is a rare, autosomal recessive disease estimated to affect approximately one in every 200,000, but its prevalence may be underestimated due to misdiagnosis. It is a chronic disease of the skin, characterized by severe inflammation, pruritus, scaling, red, and dehydrated skin. Infants born with Netherton syndrome may suffer from a failure to thrive, and it has been reported that approximately one in ten infants with Netherton syndrome die in their first year of life. Those that survive face a lifetime of skin disease challenges including red, scaly skin, hair defects and an ongoing higher than normal risk for infection and allergy.
Netherton syndrome is caused by mutations in the SPINK5 gene, which codes for the serine protease inhibitor Lympho-epithelial Kazal-type related inhibitor, or LEKTI. The function of LEKTI is to inhibit enzymes in the epidermis, which facilitate the shedding of skin cells in a process known as desquamation. When LEKTI is absent or has reduced activity, excess shedding results and the skin is sensitive, open, and appears red and scaly. This is accompanied by the detachment of the stratum corneum, leading to severe barrier dysfunction, dehydration, and potential exposure to environmental agents, such as chemicals. Netherton syndrome can range in severity from mild, such as red patchy areas of the skin, to life threatening. The degree of severity of the disease correlates directly with the extent of loss of function of LEKTI on the skin. Netherton syndrome appears shortly after birth and is most severe in the first year of an infant’s life. Survival beyond the first year is common in most cases, but the implications of the disease are a lifelong challenge.
There is no known cure for Netherton syndrome, and treatment options are limited.
The pathophysiology of Netherton syndrome is depicted below:
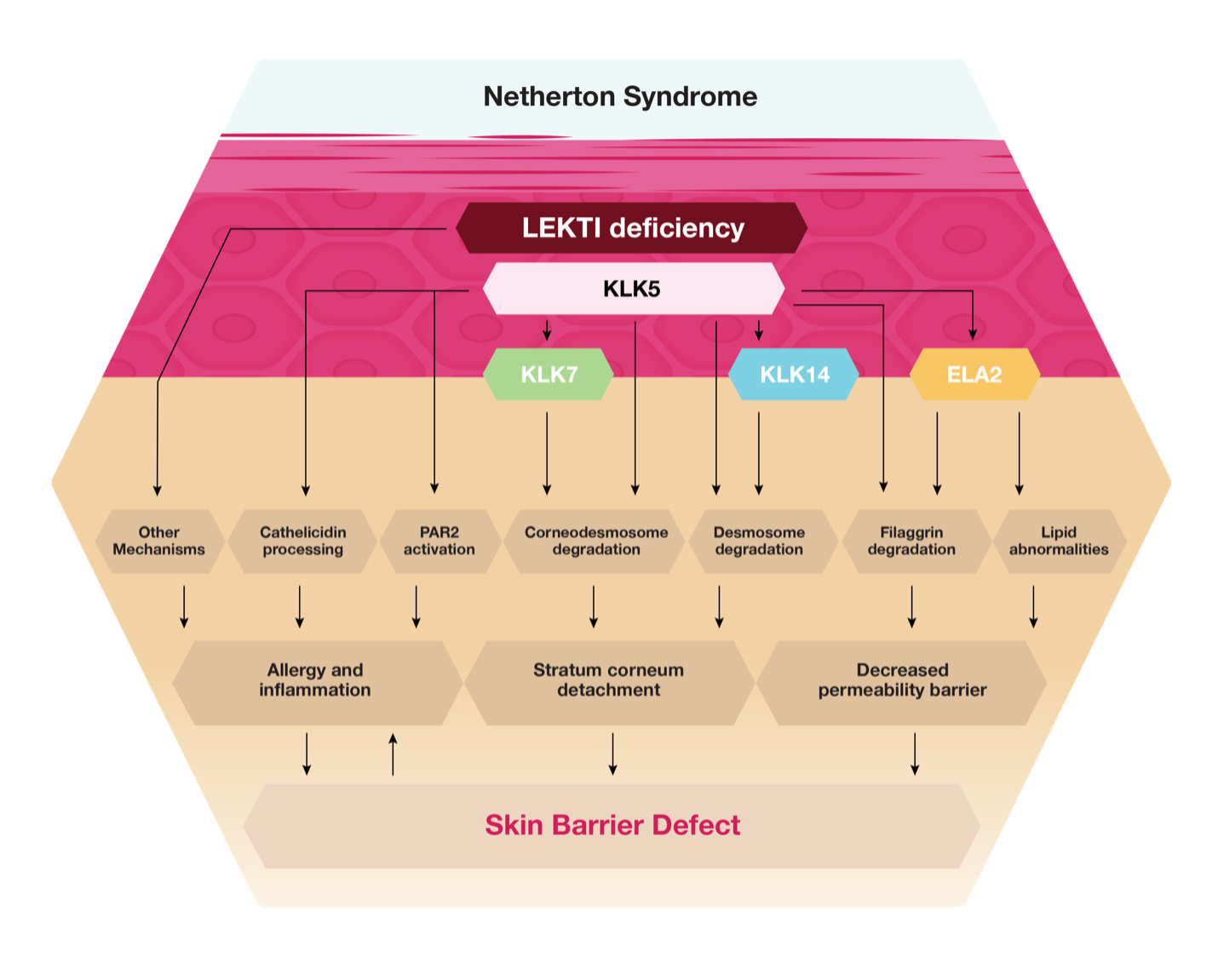
ATR-12 is our proprietary drug candidate that contains a novel strain of S. epidermidis that has been genetically modified to express and secrete an active fragment of the full-length protein LEKTI. It has also been engineered to be auxotrophic, meaning that it requires the D-alanine nutrient in its formulation to survive and propagate. This provides an additional level of safety against potential systemic infection. The topical application of ATR-12 is intended to address the underlying cause of Netherton syndrome, by replacing deficient LEKTI with an active segment of human recombinant LEKTI, or rhLEKTI-D6, to counter the dysregulated skin serine protease activity observed in Netherton syndrome patients. The uncontrolled serine protease activity leads to a profound skin barrier defect and the release of pro-inflammatory and pro-allergic mediators by keratinocytes and immune cells. We believe ATR-12 has the potential to be the first therapy to cure and effectively treat this disease of the skin. In May 2020, we received Rare Pediatric Disease Designation from the FDA for ATR-12. In January 2023, the FDA cleared a first-in-human trial for ATR-12.
ATR-04
Discovery
Disc.
Preclinical
P.C.
Phase 1
Ph.1
Phase 2
Ph.2
Phase 3
Ph.3
ATR-04
Cancer therapy-associated rash

Targeted cancer therapies have produced significant treatment advances for patients diagnosed with a variety of tumor types, but they are also associated with unique dermatologic toxicities that may hamper treatment efforts and cause significant physical and psychological discomfort for patients. One such class of targeted cancer therapy includes EGFR inhibitors (EGFRi agents). In the skin, EGFR regulates multiple functions including proliferation, adhesion and migration, survival, and differentiation. Consequently, EGFR inhibition in the skin results in adverse skin reactions, which make it difficult for patients to stay on these effective therapies. The papulopustular rash is the earliest and most common dermatologic adverse event of EGFRi treatment, often occurring in 50-80% of patients. In many cases, the rash leads to severe quality of life issues and can even lead to the interruption or cessation of the EGFRi treatment.
The current standard of care for rash treatment in patients undergoing EGFRi typically includes skin moisturizers, topical steroids, and doxycycline that are administered prophylactically from the start of EGFRi therapy and are continued throughout the entire treatment period. If the rash continues to advance, oral steroids and/or antibiotics are administered. However, there are known systemic adverse events associated with these adjunctive therapies, and we believe that physicians and patients try to limit their use, particularly with oral antibiotics. Given the high incidence rate of rash that continues with these patients, as well as the concerns related to potential impacts of antibiotics on these therapies, we believe there is a clear unmet medical need for additional safe and effective adjunctive therapies for addressing papulopustular skin rash.
Additionally, recent studies have suggested that Interleukin-36 gamma, or IL-36g and S. aureus are linked to and play a significant role in the rashes experienced by patients treated with EGFRi’s. IL-36g, is elevated in the skin of patients undergoing EGFRi therapy. IL-36g expression is induced by EGFR inhibition, and Cutibacterium acnes synergistically induces IL-36g in the skin and subsequently IL-8 and NF-kB, which leads to cutaneous neutrophilia. Additional studies have shown that S. aureus is elevated in patients undergoing EGFRi therapy. Mechanistically, EGFRi therapy impairs host defense: impaired expression of antimicrobial peptides, especially against S. aureus; and lowered expression of tight junctions.
ATR-04 is our formulated, drug product candidate for the treatment of EGFRi associated rash. It includes a novel auxotrophic strain of S. epidermidis strain that was selected from our microbial strain library, based on desired properties of IL-36g reduction and inhibition of S. aureus and its biofilms. The current lead strain is called SE484. We then genetically engineered SE484 to be auxotrophic tor D-alanine and to create our drug product candidate, ATR-04. SE484 was chosen from our microbial library based on key characteristics such as inhibition of IL-36g as well as its effect against S. aureus. Together, we expect these mechanisms of action to lead to significant reductions in rash severity among patients undergoing EGFRi therapy.
We believe that ATR-04 has the potential to address current limitations to treatment of EGFRi-associated rash:
- Reduced antibiotic use. From our surveys of clinicians and key opinion leaders, practitioners are reluctant to prescribe systemic antibiotics to patients undergoing EGFRi therapy. We believe ATR-04 would reduce the need for antibiotics in these patients and lead to fewer adverse events due to EGFRi and antibiotic use.
- Better EGFRi compliance. Up to 20% of patients undergoing EGFRi therapy discontinue due to adverse events, primarily due to rashes. We believe we can reduce discontinuation rate in patients undergoing EGFRi therapy and thus increase compliance.
- Better quality of life. Many patients on EGFRi therapy report a poor quality of life due to adverse events and papulopustular rashes. Current treatment options fail to adequately reduce these adverse events. We believe ATR-04 therapy in patients undergoing EGFRi therapy will have reduced rash severity and thus a higher quality of life.
ATR-01
Discovery
Disc.
Preclinical
P.C.
Phase 1
Ph.1
Phase 2
Ph.2
Phase 3
Ph.3
ATR-01
Ichthyosis vulgaris

Ichthyosis vulgaris (IV) is a chronic, xerotic, scaly skin disease with an estimated incidence and prevalence of 1 in 250, which gives a total patient population of 1.3 million in the United States. Clinical features of IV usually appear at around 2 months of age and include generalized xerosis and fine, white to gray scales that are prominent on the abdomen, chest, and extensor surfaces of the extremities.
Ichthyosis vulgaris is an autosomal semidominant disease caused by loss-of-function mutations in the gene encoding filaggrin. Filaggrin is an essential structural protein that is derived from profilaggrin, which breaks down into individual filaggrin units in the stratum corneum. These reinforce the skin barrier by binding to keratins and other intermediate filament proteins in the keratinocyte cytoskeleton. Many studies have identified loss-of-function mutations in FLG in IV patients, and these mutations are associated with disorganized keratin filaments, skin barrier defects and microfractures in the stratum corneum leading to enhanced percutaneous allergen sensitization. Moreover, filaggrin and its breakdown products have significant additional functions in the skin including moisturizing the skin (via hygroscopic amino acids or “natural moisturizing factors”), effecting production of antimicrobial molecules (particularly against S. aureus) and maintaining both a beneficial lipid profile and pH in the skin. There are few effective therapies for the treatment of IV.
ATR-01 is our drug product candidate intended to treat ichthyosis vulgaris. We are developing ATR01 as a novel treatment modality for IV that directly addresses the disease pathophysiology. ATR-01 consists of FLG5-6 functional unit of the human FLG protein with an attached cell penetrating peptide. The goal is to supplement the skin with stable delivery of hFLG via topical application and deeper skin penetration with a cell penetrating peptide.
Bayer Collaboration
Discovery
Disc.
Preclinical
P.C.
Clinical
Clinical
Bayer Collaboration

In December 2019, we entered into a Joint Development Agreement with Bayer for the joint development of certain strains selected from our proprietary microbial library for consumer health product candidates. We and Bayer have agreed to cooperate in the identification and characterization of S. epidermidis strains for topical formulations. After screening through hundreds of strains, we and Bayer have selected two particular strains to move forward with in vitro and ex vivo characterization.
